Food labeling is required for most prepared foods, such as vegetable oils, spices, cereals, canned and frozen foods, snacks, desserts, drinks or dietary supplements.
Nutrition labeling for raw produce (fruits and vegetables) and fish is voluntary.
A food shall be deemed to be misbranded if its labeling is false or misleading in any particular, or unless it bears a label containing the name and place of business of the manufacturer, packer, or distributor, and an accurate statement of the quantity of the contents in terms of weight, measure, or numerical count.
Don’t lose your shipment. Make sure the labels of the products you manufacture, export, import or distribute, are FDA compliant. Save time and money, now.
Manufacturers with less than $10 million in annual food sales must now comply.
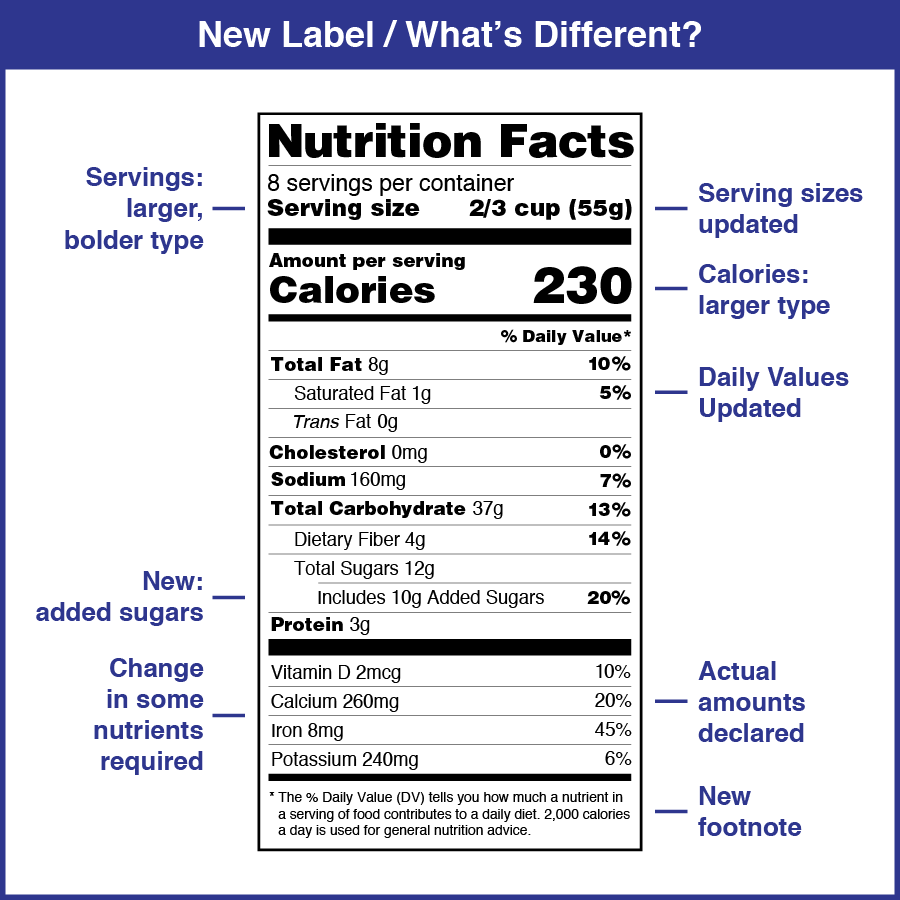
The new Nutrition Facts label is designed to provide information that can help consumers make informed choices about the food they purchase and consume.
The changes include modifying the list of required nutrients that must be declared on the label, updating serving size requirements, and providing a refreshed design.
Vitamin D and Potassium are added to the Nutrition Facts label because Vitamin D is important for its role in bone health, and Potassium helps lower blood pressure. Calcium and Iron are already required and will continue to be on the label. The actual amount (in milligrams or micrograms) in addition to the %DV must be listed for Vitamin D, Calcium, Iron, and Potassium.
“Added Sugars” in grams and as a percent Daily Value (%DV) is now required on the label.
What label statements are required on the containers and packages of dietary supplements?
▪ Identity (name of the dietary supplement).
▪ Net quantity of contents (amount of the dietary supplement).
▪ Nutrition labeling.
▪ Ingredient list.
▪ Name and place of business of the manufacturer, packer, or distributor.
Additional information includes domestic telephone number to report any health issues associated with the use of the product.
▪ You must list dietary ingredients without Reference Daily Intakes (RDIs) or Daily Reference Values (DRVs) in the “Supplement Facts” panel for dietary supplements. You are not permitted to list these ingredients in the “Nutrition Facts” panel for foods.
▪ You may list the source of a dietary ingredient in the “Supplement Facts” panel for dietary supplements. You cannot list the source of a dietary ingredient in the “Nutrition Facts” panel for foods.
▪ You are not required to list the source of a dietary ingredient in the ingredient statement for dietary supplements if it is listed in the “Supplement Facts” panel.
▪ You must include the part of the plant from which a dietary ingredient is derived in the “Supplement Facts” panel for dietary supplements. You are not permitted to list the part of a plant in the “Nutrition Facts” panel for foods.
▪ You are not permitted to list “zero” amounts of nutrients in the “Supplement Facts” panel for dietary supplements. You are required to list “zero” amounts of nutrients in the “Nutrition Facts” panel for food.
Most Supplement Facts labels fall under these categories:


Conventional Food
Nutrition Facts
Dietary Supplements
Supplement Facts
Standard
English Only

The packaging and labeling of food is subject to regulation in most regions or jurisdictions, both to prevent false advertising and to promote food safety.
Don’t take a chance assuming that regulations in the United States, Canada, Mexico, Brazil, Australia, India, South Korea, China, Japan, Russia, European Union and United Kingdom, are the same or similar.
FRANÇAIS | FRENCH
Parlez-vous français?
ESPAÑOL | SPANISH
Hablas español?
DEUTSCH | GERMAN
Sprichst du deutsch?
NEDERLANDS | DUTCH
Spreek je Nederlands?